Radioactivity
Atomic nuclei that are not stable, tend to approach stable configuration(s), by the process of radioactivity. Atoms are radioactive because the ratio of neutrons to protons is not ideal. Through radioactive decay, the nucleus approaches a more stable neutron to proton ratio. Radioactive decay releases different types of energetic emissions. The three most common types of radioactive emissions are alpha particles, beta particles, and gamma rays. Fission also is a form of radioactive decay.
Alpha (a) decay occurs when the neutron to proton ratio is too low. Alpha decay emits an alpha particle, which consists of two protons and two neutrons. This is the same as a helium nucleus and often uses the same chemical symbol 4He2. Alpha particles are highly ionizing (e.g. deposits energy over a short distance). Since alpha particles lose energy over a short distance, they cannot travel far in most media. For example, the range of a 5 MeV alpha particle in air is only 3.5 cm. Consequently, alpha particles will not normally penetrate the outermost layer of the skin. Therefore, alpha particles pose little external radiation field hazard. Shielding of alpha particles is easily accomplished with minimal amounts of shielding. Examples of alpha particle emitting radio-nuclides include 238U, 239Pu, and 241Am.
238U92 à 234Th90 + 4He2.
239Pu94 à 235U92 + 4He2.
241Am95 à 237Np93 + 4He2.
After the emission of an a particle, the daughter product remaining, will be reduced by 4 in its mass number, and 2 in its atomic number, as could be verified in the examples above.

Beta (b-) decay occurs when the neutron to proton ratio is too high. The radioactive nucleus emits a beta particle, which is essentially an electron, in order to bring this to a more favourable ratio. Beta particles are less ionizing than alpha particles. The range of beta particles depends on the energy, and some have enough to be of concern regarding external exposure. A 1 MeV beta particle can travel approximately 12 feet in air. Energetic beta particles can penetrate into the body and deposit dose to internal structures near the surface. Since beta particles are less ionizing than alpha particles, greater shielding is required. Low Z materials are selected as beta particle shields to take care of X-ray emissions associated with slowing down of beta particles while they travel in a medium.
In b emission, the neutron to proton ratio is reduced by converting a neutron into proton as:
1n0 à 1p1 + e-. The electron ejected is the b particle that is released. Thus b emission results in the increase of the proton number, i.e. Z, by 1, but the mass number A is unaltered. Example of b decay: 40K19 à 40Ca20 + β-1

Gamma (g) rays are not particulate radiation like the alpha and beta, but a form of high-energy electromagnetic wave. Gamma rays are the least ionizing of the three forms discussed. A 1 MeV gamma ray can travel an average of 130 meters in air. Since gamma radiation can travel far in air, it poses a significant external radiation hazard. Further, if ingested, it may pose an internal radiation hazard. Shielding of gamma rays is normally accomplished with high atomic number materials such as lead. [Gamma rays are electro-magnetic radiations with energies higher than X-rays. X-rays are produced when electrons of an atom jump from one orbital location to another. The gamma rays are released when an atomic nucleus releases its excess energy. It is clear from this that nuclear transitions involve much larger energies than the atomic transitions. In other words, energies of nuclear origin are many (103 – 106) times greater than the energies of atomic origin].
Emission of γ-rays doesn’t change the Mass number nor Atomic number. If an atom is in exited state it comes to stable state by emitting a γ radiation.
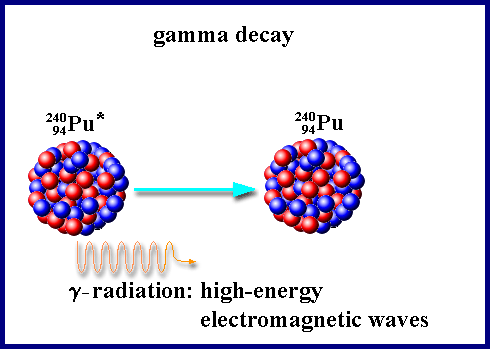
Usually after α or β decay, the product nucleus is formed in an excited state and it reaches a stable state after γ emission.
|
|
There are several other particles, like neutron, proton, 3He, deuterium, etc. that may be liberated in radioactivity. When a nucleus emits such particle(s) toward reaching a stable configuration, it is said to ‘decay’. The emitted particle is associated with the ‘mode’ of decay. Thus we have alpha decay, beta-decay, gamma decay, neutron-decay, etc. In the case of gamma emission, the nucleus changes only in its energy state. By particle emission, the nucleus changes into another, and so is said to be transmuted or converted. Thus there is a reduction in the original quantity of the substance, during decay. There is no fixed time between two consecutive emissions, but on an average, radioactive decay of a substance takes place at a rate, which is proportional to the number of atoms present, at a given time. This is expressed in a well-known differential equation, called radioactivity decay equation.
An atom becomes radioactive if its nucleus suffers instability, as said earlier. A nucleus may be radioactive due to instability set in while it was formed in nature. This is called ‘natural radioactivity’, like that of 238U. When a nucleus is ‘disturbed or excited’, say, by bombarding it with a particle or gamma rays, its state of stability is altered and the altered system will become radioactive. This is referred to as ‘induced radioactivity’ or ‘artificial radioactivity’. There could be many ways of putting a nucleus in a slightly or heavily unstable or ‘excited’ state. But the consequent radioactivity, which is a process of de-excitation, is governed by common laws. The de-excitation may take place quickly (say, in micro-seconds) or over a long period (in millions of years), in a single step or in a series of many steps. Hence when we talk of the radioactivity of a substance, we talk of the original radioactive material (parent), what fraction of it gets converted in unit time, what are the particles released (emitted), how much energy is released, what are the new materials (daughter products) formed, radioactivity features of the daughter products, and of the end-product (stable) as well. The rate at which the decay takes place is called ‘activity’.
Rate of Radioactive Decay: The nuclei of a given radioactive species have a definite probability of decaying in unit time; this decay probability has a constant value characteristic of the particular nuclide. It remains the same irrespective of the chemical or physical state of the element at all readily accessible temperatures and pressures. In a given specimen the rate of decay at any instant is always directly proportional to the number of radioactive atoms of the nuclide under consideration present at that instant. Thus, if N is the number of the particular radioactive atoms (or nuclei) present at any time t, the decay rate is given by
dN/dt = -λt
where λ, called the decay constant of the radioactive nuclide, is a measure of its decay probability in unit time. Upon integration between any arbitrary zero time, when the number of radioactive nuclei of the specified kind present is N0, and a time t later, when N of these nuclei remain, radioactive decay is seen to be an exponential process, the actual decay rate being determined by the decay constant λ and by the number of the particular nuclei present.
ln(N/N0) = -λt,
N=N0e-λt
Mean life: The reciprocal of the decay constant, represent by tm, is called as the mean life (or average life) of the radioactive species; thus,
tm=1/λ
The mean life is equal to the average life expectancy of the radioactive species.
Half life: It is defined as the time required for the number of radioactive nuclei of a given kind (or for their activity) to decay to half its initial value. Because of the exponential nature of the decay, this time is independent of the amount of the radionuclide present. It can be seen from the equations given above, that the half life is given by
t1/2=(ln 2)/λ = 0.6931/λ
or
t1/2=0.6931tm
The half life is thus inversely proportional to decay constant and directly proportional to mean life. The half-lives of known radioactive nuclides range from a small fraction, e.g., about a millionth of a second to billions of years.
Units to express radioactivity emission: There are several different units used to describe radiation and its effects. The simplest unit is that of activity which is measured in number of disintegrations per second (dps). One dps means a radioactive nucleus gives off one particle or photon in one second. This unit, in the international system of units (SI) system, is called the becquerel (Bq), which is equivalent to 1 dps. The other unit prevalent for the activity, is a Curie (Ci). 1 Ci = 3.7 ´ 1010 Bq. These units do not distinguish between alpha, beta or even gamma. These units provide an understanding of the "strength" of the radioactive sample but do not account for any of the properties of the radiation emitted. To describe the degree of hazard to people from a particular radiation requires other units.
Energy Levels: As mentioned earlier, ‘quantum mechanics’ is needed to understand and quantify atomic and subatomic features. The Quantum Theory recognizes restrictions in the levels of energy acquired by a system. The electrons in their orbits, or the nucleons in the shells are filled following such restrictions. The energy of an electron depends on its orbit, and only certain orbits are permitted by nature. Similarly the nucleons within a nucleus occupy different energy states that are permitted. We understand from this that an electron or a nucleon cannot go to any arbitrary energy level. Thus when the system is specified, the allowed energy states get specified. These are known as discrete energy levels. Transition from one such level to the next lower level will involve release of energy that is exactly the difference between these two levels, and cannot be a fraction of the same. The energy is said to get ‘quantised’. The electron orbits permitted for an atom are characteristic of that (species of) atom, and when an electron jumps from one orbit to the lower one, X-ray, called ‘characteristic X-ray’, with energy equal to the difference between the energies associated with the two orbits emerges. This helps even identifying the atom from which the X-rays ejected. Similar nature applies to nuclei too. Though there are no orbits inside a nucleus for the nucleons to move around, they have their energy states. A given radioactive gamma-emitting nucleus will eject gamma rays (called gamma quanta) characteristic of the nucleus. The energies taken by the nucleons within an unexcited nucleus are called bound levels.
Similarly, a nucleus could be raised (excited) in its internal energy only to certain permitted levels. These levels are called excited levels. This varies from nucleus to nucleus, but is fixed for a given nucleus. When the nucleus is unexcited, it is said to be in its ground state. The separation between two level energies decreases as the energy increases. When a nucleus is excited to a particular level, it de-excites to reach the ground state by emitting a neutron, gamma quanta, or any other particle. Fission also is such a process. The de-excitation could be in a single step, or in multiple steps involving a series of particle emissions or gamma or both.
