Atomic Nucleus
An atom consists of a centrally located nucleus surrounded by electrons revolving in certain physically permitted orbits. The nucleus itself is made up of neutrons and protons, collectively called nucleons.
The neutrons are electrically neutral, the protons positive (with 1.6 x 10-19 coulomb of charge) and the electrons negative with the same magnitude of charge. The nuclear dimension is in the range of 10-13 – 10-12 cm, while the atomic dimension is about 10-8 cm. That is, the diameter of an atom is over 10,000 times the diameter of its nucleus. 1 cm is nearly the width of our little finger. The atomic diameter is about one hundred millionth the width of our little finger. The nuclear size is 1 billionth the width of our little finger. This nuclear range is called a short-range, wherein the nucleons attract each other with a force, much larger than the electrostatic repulsion between the positively charged protons. This means, two neutrons attract each other though they are neutral; two protons attract each other though they are both positively charged; a proton and a neutron attract each other though they are not of opposite charges. In other words the nucleons bind themselves together when they happen to come closer than 10-12 cm. Within this range, the closer they are the stronger their bond is.
The electron orbit need not be circular, but may be oval in shape. Generally, there are as many electrons around the nucleus as there are protons in the nucleus, thus making the atom as a whole, neutral. Please note that the atomic neutrality is with respect to a point sufficiently away from the atom, since the influences of electrons and protons cancel out. The respective charge would be felt, very close to the electron or the nucleus. If one or more electrons are removed from the orbit, the atom has a net positive charge, and is said to be positively ionized. Similarly, if electrons are added to the orbits, the atom becomes negatively ionized. See figure below:
|
Neutral atom |
Positively ionized atom |
Negatively ionized atom |
|
|
|
|
|
|
||
The number of protons (Z) is called the atomic number and the total number (A) of nucleons in a nucleus is called the atomic (or nuclear) mass number. Occasionally the number of neutrons (A-Z) is represented as N. Atoms are classified as isotopes, isotones, and isobars based on the nuclear contents, as below:
|
Isotopes |
Isotones |
Isobars |
|
Nuclei with same Z but differing in N |
Nuclei with same N but differing in Z |
Nuclei with same A but differing in N, Z or both |
|
Example: U-235, U-238 |
Example: U-238, Np-239 |
Example: U-238, Np-238, Pu-238 |
There are about 266 stable nuclides, and 65 radioactive nuclides in nature. A radioactive nucleus is one in an unstable configuration. It approaches stable configuration(s), by emitting radiation in the form of gamma energy, or particles like alpha, beta, neutron, etc., or by splitting into fragments. The internal energy state of a nucleus is responsible for the stability of a nucleus, and is affected by the number of protons and number of neutrons in the nucleus. The following table gives the distribution of the stable nuclei with respect to even/odd combinations of Z and N. It shows that an even-even combination of Z and N gives high stability, and odd-odd the least.
Stable nuclides
|
A |
Z |
N |
Number |
|
Even |
Even |
Even |
159 |
|
Odd |
Even |
Odd |
53 |
|
Odd |
Odd |
Even |
50 |
|
Even |
Odd |
Odd |
4 |
|
Total |
266 |
||
Further, Z or N = 2,8,20,50,82,126 gives high stability to a nucleus, and these numbers are referred to as magic numbers. If Z and N of a nucleus happen to be individually equal to magic numbers, the configuration is called doubly magic leading to exceptional stability. The magic numbers are not mythical, though they appear to be so. A nucleus is understood to be formed in a shell-structure, with each shell having a ‘capacity’ for filling with neutrons or protons. Each shell gets ‘closed’ when it is filled to full capacity. Quantum mechanical arguments show that the capacities of different shells correspond to the (so called) magic numbers. If all the shells of a nucleus are closed, it becomes very stable.
As said, the stability of a nucleus is influenced by the numbers of protons and neutrons in it. Hydrogen 1H1 has one proton and no neutron. This is the only nucleus without a neutron. An isotope of hydrogen, viz. heavy hydrogen 2H1, some times called ‘deuterium 2D1’, has a proton and a neutron. Light stable nuclei, except 1H1, have generally equal number of protons and neutrons. As the nucleus becomes heavier, the N/Z ratio, or the ‘neutron-to-proton ratio’ of stable nuclides increases. As said earlier, the nuclear short-range attractive force is the same between any type of nucleons. As the number of protons increases, the electric repulsive force becomes more and more significant. Neutrons have no electric repulsion but only attraction. Hence adequate presence of neutrons will help overcome the average repulsive force per nucleon in the nucleus. Thus stability of a high-Z nucleus demands that the number of neutrons well exceeds the number of protons. In other words, more neutrons than protons are needed to support the binding of the positively charged protons within the nucleus, as the proton number gets larger. One must not jump to the conclusion that the more the neutrons, the more stable the nucleus is. The picture below shows the numbers of neutrons and protons in stable nuclei and illustrates how the N/Z ratio deviates from unity. The black squares indicating stable nuclides form an approximate line called the ‘Stability Line’. Sometimes it is called the ‘beta stability line’.
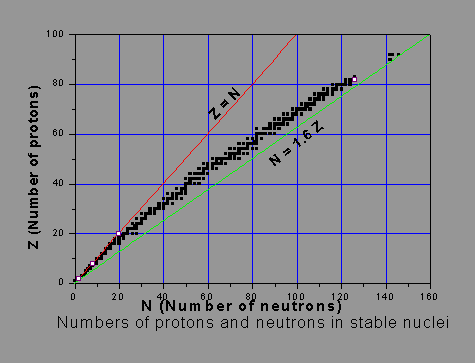
Excessive or inadequate number of neutrons, as compared to the stability-line, leads to instability of the nucleus, leading to its radioactivity. Some of the very stable (magic) nuclei (e.g. lead, with 82 protons and 126 neutrons), are shown as open squares.
The masses of sub-atomic particles are often expressed in ‘atomic mass unit (amu)’.
1 amu = 1.6606 x 10-27 kg. The masses of proton, neutron and electron are given below:
|
Particle |
Mass (amu) |
|
Proton |
1.007277 |
|
Neutron |
1.008665 |
|
Electron |
0.0005486 |
This shows that a neutron is slightly heavier than a proton, and an electron is lighter by a factor of about 1840.
According to Einstein, energy and mass are related. Energy = mass ´ c2, where c is the speed of light = 2.998 ´ 108 m/s. The unit of energy often chosen in nuclear physics is an electron-volt (eV). 1 eV is the energy associated with an electron moving across a potential difference of 1 Volt. 1 million eV is denoted as MeV. 1 MeV = 1.602 x 10-13 Joules. 1 amu = 931.5 MeV. These units will be extensively used in discussions below.